
By Miriam Dabrowa, WILDDESIGN
The Chinese medical device market, according to statistics and economic forecast, is an increasingly attractive place to invest because of many interrelated factors, described more extensively in the first article in this series, Chinese Medical Device Market: Opportunities And Challenges.
But, before entering this promised land of annual growth forecasts in excess of 15 percent, device makers need to do their homework. While technical requirements obviously must be followed, companies must also gauge how cultural and language barriers may affect market entry.. In light of these considerations, this article outlines steps to ensure the fastest, smoothest market entry possible.
1. Research The Market (Or Consult Professionals)
Make sure there is a need for your product on the Chinese market. The needs of Chinese consumers and hospitals change regularly, so you need to observe the trends and decide if your product is competitive enough or fills a niche. Keep in mind that Chinese public hospitals are encouraged to buy local products, and that they are allowed to purchase Western equipment only if there is no suitable alternative offered by a Chinese producer. Thus, device manufacturers may be more likely to find success offering innovative and high-quality products.
Make sure there is a need for your product on the Chinese market. The needs of Chinese consumers and hospitals change regularly, so you need to observe the trends and decide if your product is competitive enough or fills a niche. Keep in mind that Chinese public hospitals are encouraged to buy local products, and that they are allowed to purchase Western equipment only if there is no suitable alternative offered by a Chinese producer. Thus, device manufacturers may be more likely to find success offering innovative and high-quality products.
Also, it’s a good idea to conduct at least basic research, because product functions held in high regard by Western medical staff may not hold equal appeal among their Chinese counterparts. Interviews with local key opinion leaders — and potential users — can be crucial to a company’s market entry decision. Furthermore, always seek reputable research agencies, recognized as knowledgeable in the Chinese marketplace, to obtain the most precise and accurate data.

2. Who Is Who?
Identify the main players — not only direct competitors, but government bodies accountable for medical device registration and certification. This latter step is especially important, as medical device manufactures may discover Chinese procedures, and the overall registration process, to be more complicated and time-consuming than in other nations.
Identify the main players — not only direct competitors, but government bodies accountable for medical device registration and certification. This latter step is especially important, as medical device manufactures may discover Chinese procedures, and the overall registration process, to be more complicated and time-consuming than in other nations.
- Chinese Food and Drug Administration (CFDA) — The most important ministerial-level institution when it comes to starting a medical business in China. It is responsible for registration of medical devices for the Chinese market, drafting new regulations and policy plans, and ensuring device safety.
- Center for Medical Device Evaluation (CMDE) — Responsible for conducting the dossier review of testing samples during the medical device registration process.
- General Administration of Quality Supervision, Inspection, and Quarantine (AQSIQ)— Responsible for mandatory safety registration, certification, and inspection of certain devices.
Be sure to regularly check these organizations’ websites — CFDA in particular — for new updates and regulations.
3. Familiarize Yourself With The Registration Process
According to CFDA regulations, “’medical devices’ refer to those instruments, equipment, tools, materials and other objects, including the software attached to them, that are designed to be used either independently or in combination on human body. These devices are used for:
According to CFDA regulations, “’medical devices’ refer to those instruments, equipment, tools, materials and other objects, including the software attached to them, that are designed to be used either independently or in combination on human body. These devices are used for:
- Prevention, diagnosis, treatment, monitoring or remission of diseases
- Diagnosis, treatment, monitoring, remission or compensation of injury or physical disability
- Research, replacement or adjustment of anatomical or physiological process
- Control of pregnancy”
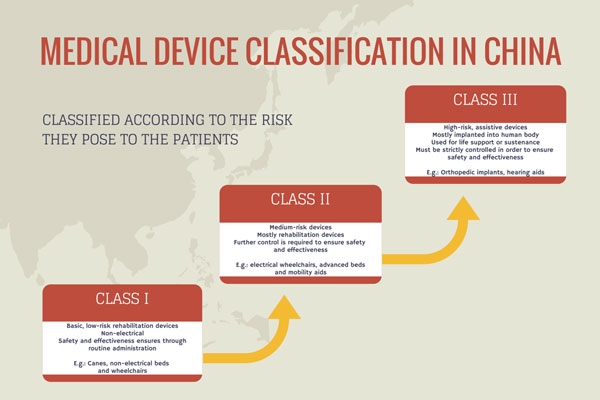
Before applying to CFDA, a device maker must specify the class of its product. There are three classes of medical devices, graded according to their risk impact. Class I consists of low-risk, low-maintenance and non-electrical devices, while Class III includes high-risk devices, usually those implanted into the human body or used for life support.
Again, the registration process can be burdensome. The minimum time period to obtain product registration is about 14 months. Advanced devices requiring clinical trials will take even longer, and approval in the country of origin is required before registration in China can take place.
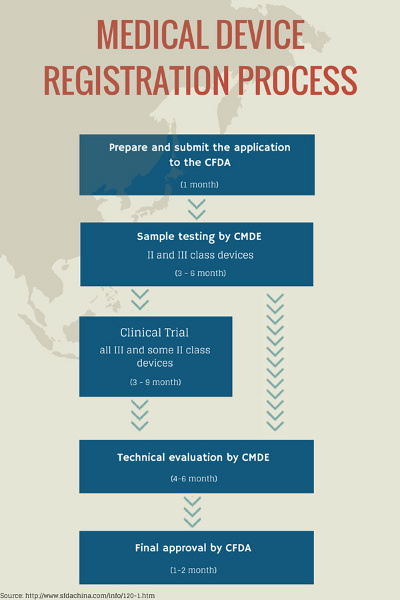
4. Develop And Design In Accordance With Chinese Device Requirements — From The Beginning
Here at WILDDESIGN, where our origins are deeply rooted in medical design, we know that product development in medical technology follows a unique path at each manufacturer.
Here at WILDDESIGN, where our origins are deeply rooted in medical design, we know that product development in medical technology follows a unique path at each manufacturer.
However, in developing products for the Chinese market, it is important to correlate the design process with CFDA regulations from the start, since the whole process is integrated. CFDA registration, much like FDA or CE approval, requires the development process, as well as the end result, to meet specific certification requirements. That means that from the beginning, you have to develop and document the design in the design history file, and the usability process in the usability engineering file.
Remember that a properly documented process, decent graphical user interface (GUI) design, and usability are the vehicles to obtaining necessary certifications faster, avoiding application rejections and costly delays, and meeting customers’ expectations.
5. Find Trustworthy Distributors And Partners
Collaborate with reliable and qualified representation agents and distributors. CFDA requires three local agents for introduction of a medical device:
Collaborate with reliable and qualified representation agents and distributors. CFDA requires three local agents for introduction of a medical device:
- Registration agent — The company that registers the product
- After-sales agent — Leads technical service and support for the product
- Legal agent — Responsible for reporting any events regarding the device that occur inside or outside China, and for handling any recall issues or other regulatory matters
Although some distributors are qualified to act as local agents, as well, it is highly recommended to divide these two (legal and business) functions to avoid potentially difficult and costly entanglements. This is because a partner serving in both roles must sign and officially stamp documentation giving up their responsibilities if you decide the business arrangement with that distributor/agent is unsatisfying.. Also, because of the size and fragmentation of the Chinese market, very few distributors offer nationwide coverage, meaning device manufacturers likely need to partner with several distributors, or cooperate with a partner that has access to a wider business network.
A device manufacturer also may set up its own legal structure in China, called a wholly foreign owned enterprise (WFOE), and administrate the entire business itself.
Entering Chinese market might seem complicated but, if your market research and strategy connect well, your company soon may enjoy rewarding growth and business development in this very demanding, yet highly promising, trade market.
No comments:
Post a Comment